- Home
-
- ZEROLab
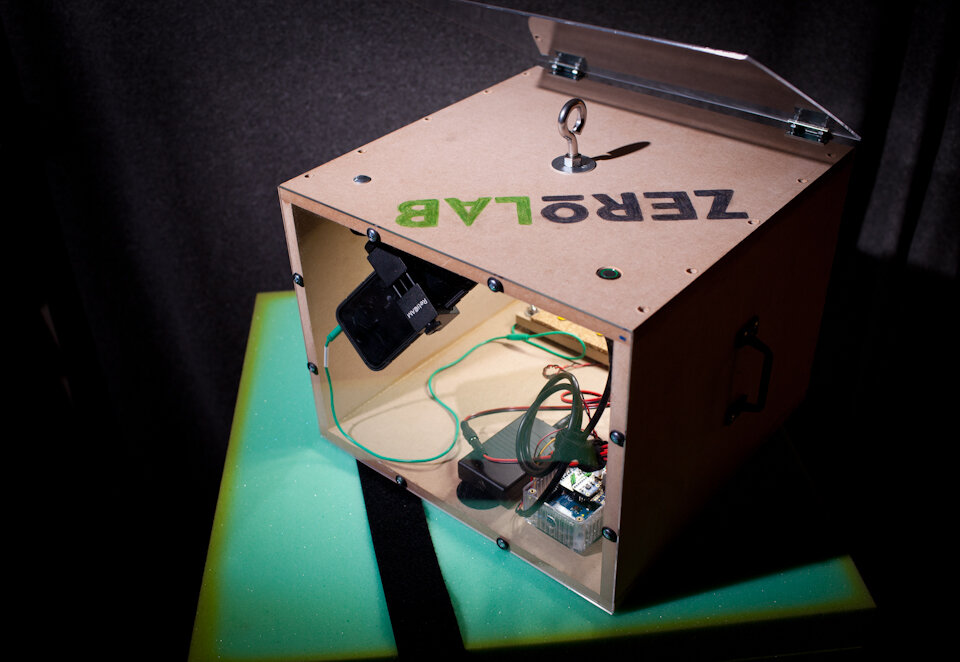
ZEROLab
“ZEROLAB” Microgravity Electrophysiology
 ABSTRACT
ABSTRACT
In 1957 the Soviet Union showed that an animal could survive exposure to a zero gravity environment, however, we have yet to fully understand how the nervous system of complex organisms responds to these situations. Classifying network-level changes in two and three order neural circuits could have profound implications for long duration space flight and celestial colonization. Furthermore, neurological diseases underpinned by these circuits may exhibit alterations in reduced gravity, and understanding their etiology, progression, and response has the potential to inspire more efficacious countermeasures. To answer some of these questions and ignite further microgravity investigations, I developed a low-cost, open-source electrophysiology lab that can record neural activity during free fall.
 INTRODUCTION
INTRODUCTION
The study of biology in zero gravity, while incredibly important to human space exploration, is notoriously difficult and expensive to pursue. The range of options are in-part limited to airplanes equipped for parabolic flight (Paul et al., 2011), elaborate drop towers (Wiedemann, Rahmann, & Hanke, 2003), random positioning machines for cell culture (Wuest, Richard, Kopp, Grimm, & Egli, 2015), and outer space missions (Buckey & Homick, 2003). The most significant advancement to our understanding of the nervous system during space flight was by means of “Neurolab,” a 1998 mission aboard the Space Shuttle Columbia. While it provided incredible insight into developmental, histological, and cognitive neuroscience across several species (Buckey & Homick, 2003), direct electrophysiological measurements on living organisms were not conducted during flight. Recent collaborations between NASA and SpaceX have re-established a scientific supply chain to the International Space Station (Garcia, 2015), though it will undoubtedly take ground-based studies to direct further efforts, and substantiate existing ones.
While some physiological responses to gravity diminish after several days of exposure, many can persist and are inherently problematic for humans, as we have developed and evolved in a world with continuous gravitational forces. Multisensory integration can be unreliable in anomalous conditions such as space flight (Clément & Reschke, 2009), and promote symptoms that are homologous to those well-described in literature concerning sensory processing disorder (Miller, Nielsen, Schoen, & Brett-Green, 2009). The primary gravity sensors in humans exist in the vestibular system and begin integrating with other sensory information (i.e. proprioceptive, visceral, visual) in second order brainstem neurons. At this level, incoming efference copies of motor signals also combine with internal estimates of body position, and altogether, these inputs instruct higher order function based on an internalized model of gravity (Holmes & Spence, 2004; Lacquaniti et al., 2014). Schematizing this gravity model and understanding associated neuronal phenomena, including single unit and local field potential activity, could be important steps in answering broad questions related to gravitational neurophysiology.
Crickets have long served as a model organism for gravitational research because they exhibit neurons that signal information regarding the animal’s position in the gravity field(Sakaguchi & Murphey, 1983). This signal originates from the cricket clavate receptors and is encoded by a position sensitive interneuron (PSI) located in the terminal ganglion. The PSI was investigated during the Neurolab mission for changes induced by micro- and hyper-gravity by measuring firing rate changes pre- and post-flight, and showed significant susceptibility to these conditions (Horn et al., 2002). I am, however, interested in the electrophysiological changes occurring during the transition from 1-g (i.e. earth) to 0-g (i.e. space). The cricket provides a practical model for these investigations, and could support further studies on multisensory integration.
METHODS
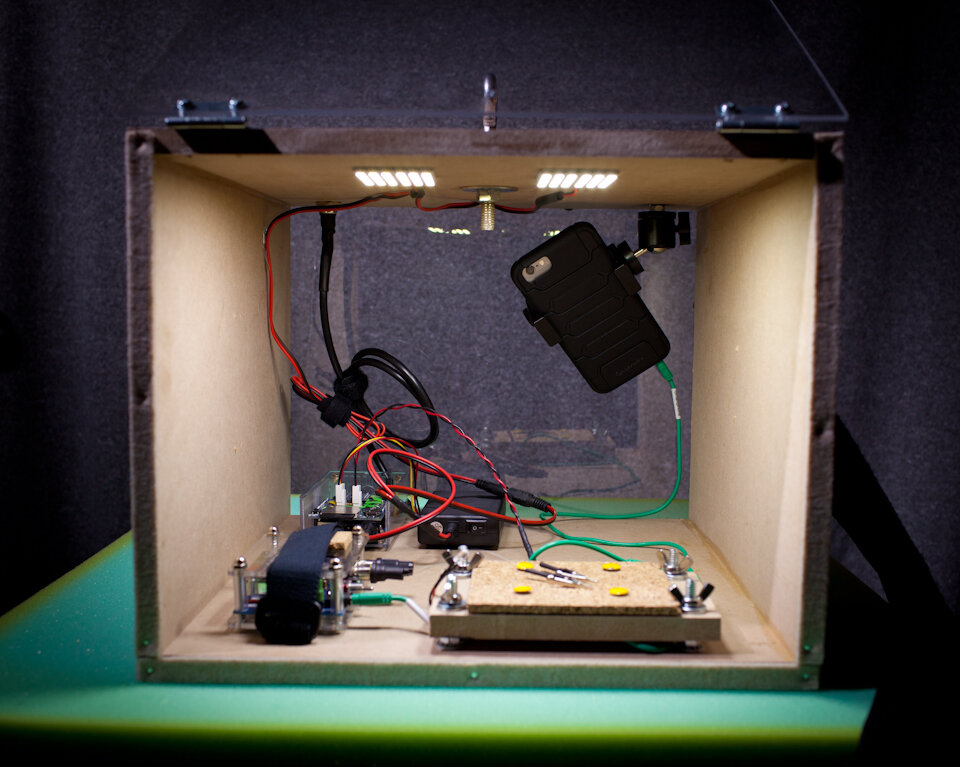
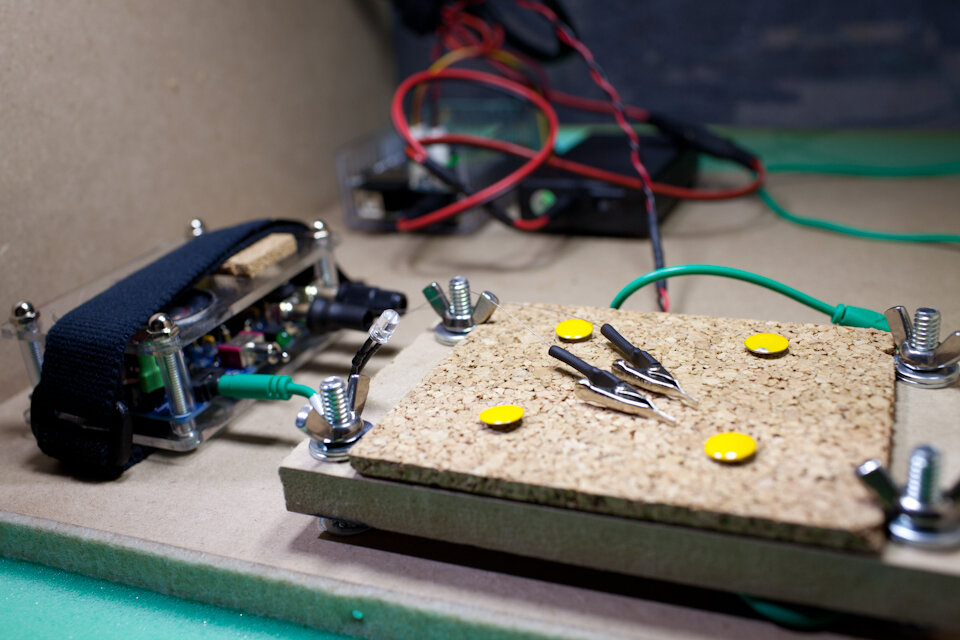
Preparation. Adult crickets (Acheta domesticus) were obtained from Petco and kept live in a small bag. Crickets were individually removed from the bag by grabbing a primary limb with coarse forceps, briefly placed on dry ice until movement ceased (5-10 seconds), and then decapitated at the head-thorax junction. Using 50-micron tungsten wire, two hook-style electrodes were fashioned with one placed on a ventral connective nerve (recording wire) and the other within the abdomen (reference wire) following previously reported methods (Dupuy et al., 2012).Recording. Electrodes were connected to a Backyard Brains SpikerBox. The extracellular signal is internally band pass filtered in this unit (338–1,129 Hz) and was connected to the microphone input of an Apple iPhone 6 with a Backyard Brains audio cable. Using the native Camera application on the iPhone, video and audio was captured at 240 frames per second (“slo-mo” feature), and at 44.1k samples per second, respectively. Data from a triple-axis accelerometer (Analog Devices ADXL335) obtained on breakout board from Adafruit was logged every 20 milliseconds using an Arduino Data Logging module from Adafruit (powered by a 12-volt battery pack, source code available: https://github.com/mattgaidica/ZEROLAB). Accelerometer data was retrieved on a removable SD flash memory card. An LED was placed within the video frame in order to sync the recording of the accelerometer data with the electrophysiology data.
The audio recording during freefall was used to identify action potentials. You can hear (volume up) when the gravity sensors respond to the microgravity stimulus.
Analysis. All data was imported to MATLAB via standard functions. The audio recording for each trial was saved to a DDT file and analyzed in Plexon’s Offline Sorter utility to discriminate individual units. Neural timestamps were then exported to an NEX file to be used for analysis in MATLAB.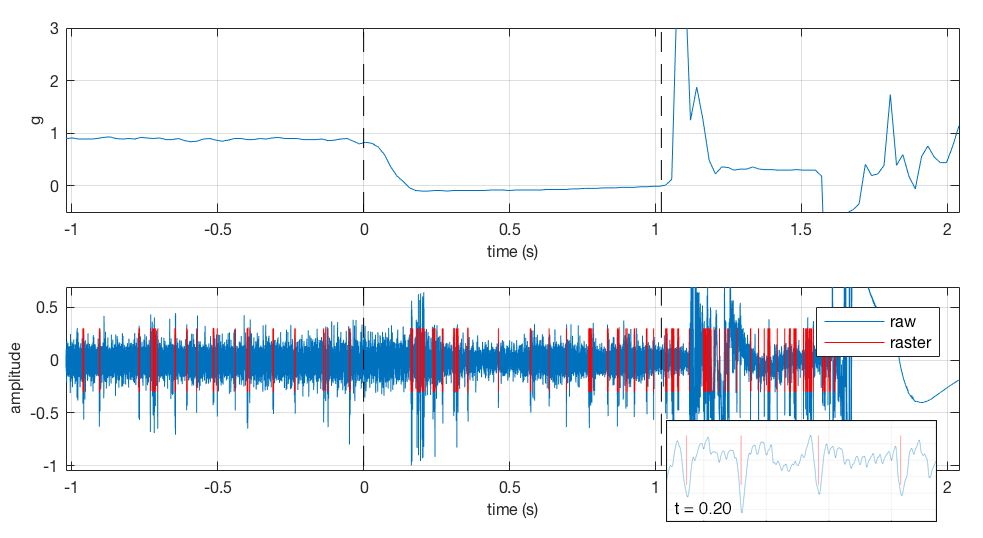 Sample of Neuronal Activity Modulation During Free-fall (~0-1s)
Sample of Neuronal Activity Modulation During Free-fall (~0-1s)
BIBLIOGRAPHY
Buckey, J. C., & Homick, J. L. (2003). The Neurolab Spacelab Mission: Neuroscience Research in Space: Results from the STS-90, Neurolab Spacelab Mission. doi:10.1001/archneur.62.8.1314-b
Clément, G., & Reschke, M. (2009). Neuroscience in Space. American Journal of Neuroradiology. doi:10.3174/ajnr.A1345
Dupuy, F., Steinmann, T., Pierre, D., Christides, J.-P., Cummins, G., Lazzari, C., … Casas, J. (2012). Responses of cricket cercal interneurons to realistic naturalistic stimuli in the field. Journal of Experimental Biology, 215(14), 2382–2389. doi:10.1242/jeb.067405
Garcia, M. (2015). Dragon Reaches Space , Headed for Friday Station Rendezvous. Retrieved from https://blogs.nasa.gov/spacestation/2015/04/14/dragon-reaches-space-headed-for-friday-station-rendezvous/
Holmes, N. P., & Spence, C. (2004). The body schema and multisensory representation(s) of peripersonal space. Cognitive Processing, 5(2), 94–105. doi:10.1007/s10339-004-0013-3
Horn, E., Agricola, H., Böser, S., Förster, S., Kämper, G., Riewe, P., & Sebastian, C. (2002). Crickets in space: Morphological, physiological and behavioral alterations induced by space flight and hypergravity. Advances in Space Research, 30(4), 819–828. doi:10.1016/S0273-1177(01)00642-1
Lacquaniti, F., Bosco, G., Gravano, S., Indovina, I., La Scaleia, B., Maffei, V., & Zago, M. (2014). Multisensory integration and internal models for sensing gravity effects in primates. BioMed Research International, 2014, 1–10. doi:10.1155/2014/615854
Miller, L. J., Nielsen, D. M., Schoen, S. A., & Brett-Green, B. A. (2009). Perspectives on sensory processing disorder: a call for translational research. Frontiers in Integrative Neuroscience, 3(13), 22. doi:10.3389/neuro.07.022.2009
Paul, A.-L., Manak, M. S., Mayfield, J. D., Reyes, M. F., Gurley, W. B., & Ferl, R. J. (2011). Parabolic flight induces changes in gene expression patterns in Arabidopsis thaliana. Astrobiology, 11(8), 743–58. doi:10.1089/ast.2011.0659
Sakaguchi, D. S., & Murphey, R. K. (1983). The equilibrium detecting system of the cricket: Physiology and morphology of an identified interneuron. Journal of Comparative Physiology ??? A, 150(2), 141–152. doi:10.1007/BF00606364
Wiedemann, M., Rahmann, H., & Hanke, W. (2003). Chapter 24 Gravitational impact on ion channels incorporated into planar lipid bilayers. Membrane Science and Technology, 7(C), 669–697. doi:10.1016/S0927-5193(03)80048-7
Wuest, S. L., Richard, S., Kopp, S., Grimm, D., & Egli, M. (2015). Simulated Microgravity : Critical Review on the Use of Random Positioning Machines for Mammalian Cell Culture,2015.
